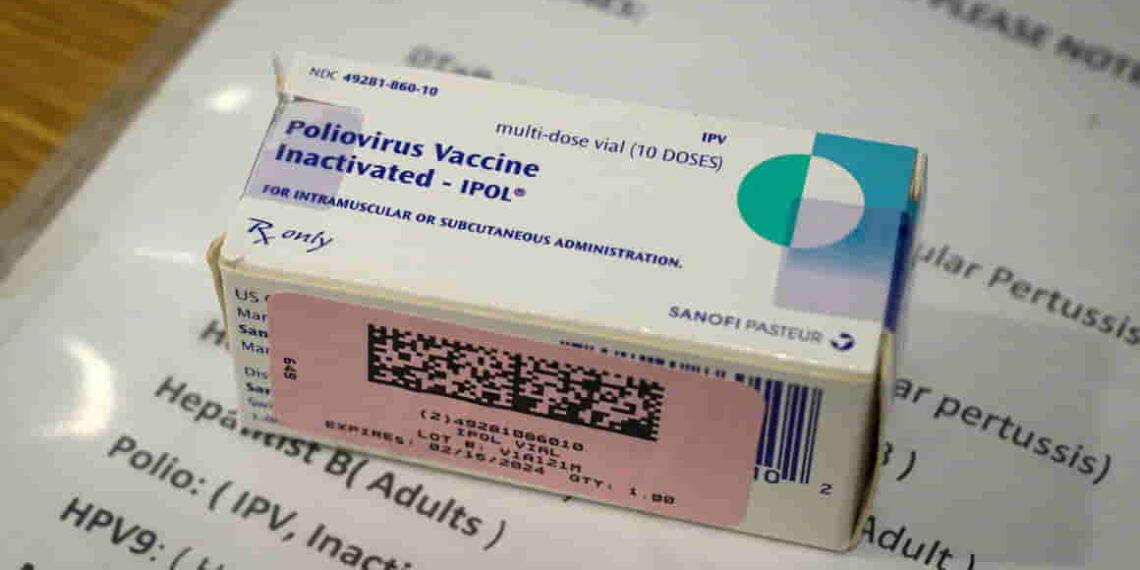
Kennedy’s Lawyer Urges FDA to Revoke Polio Vaccine Approval
A lawyer associated with Robert F. Kennedy Jr., Aaron Siri, has filed a petition asking the U.S. Food and Drug Administration (FDA) to revoke approval for the polio vaccine. This move, initiated in 2022 on behalf of the nonprofit Informed Consent Action Network (ICAN), has drawn significant attention and controversy. ICAN challenges vaccine safety and mandates, reflecting a skeptical stance on vaccination.
Trump and Kennedy’s Vaccine Views
President-elect Donald Trump has praised the polio vaccine as “the greatest thing,” yet expressed openness to reconsidering certain vaccines for children if deemed dangerous or not beneficial. In contrast, Kennedy, Trump’s pick to lead the Department of Health and Human Services (HHS), has emphasized informed choice over vaccine mandates. “People ought to have a choice, and that choice ought to be informed by the best information,” Kennedy stated in a recent interview with NBC News.
If confirmed as head of HHS, Kennedy would oversee the FDA and could potentially influence its decision-making process regarding the petition. The FDA, however, has maintained that it is reviewing the petition and cannot predict when the review will be completed.
Senate Leader’s Warning
Senate Minority Leader Mitch McConnell, a polio survivor, issued a stern warning, highlighting the life-saving impact of the polio vaccine. “Efforts to undermine public confidence in proven cures are not just uninformed—they’re dangerous,” McConnell said, advising Kennedy to distance himself from such controversies.
The Impact of Polio Vaccines
Polio vaccination is widely regarded as one of the most significant public health achievements. In the 1950s, polio paralyzed and killed thousands annually. The introduction of vaccines dramatically reduced its prevalence, bringing the world close to eradicating the disease.
Siri’s petition demands a “properly controlled and properly powered double-blind trial” to assess the inactivated polio vaccine’s safety. Critics argue this is unnecessary and misrepresents vaccine safety. Dr. Paul Offit of Children’s Hospital of Philadelphia countered, “You’re substituting a theoretical risk for a real risk. The real risks are the diseases.”
The Centers for Disease Control and Prevention (CDC) supports the safety of the inactivated polio vaccine, noting no serious adverse events have been documented, except rare allergic reactions.
Different Polio Vaccines and Risks
The United States transitioned from the oral polio vaccine (OPV), which uses a weakened live virus, to the inactivated polio vaccine (IPV) due to safety concerns. OPV carries a slight risk of causing paralysis but remains essential in regions where polio still circulates. IPV, administered via injection, eliminates this risk but does not create mucosal immunity, meaning it doesn’t prevent the virus from infecting or being shed by vaccinated individuals.
The Spread of Polio
Poliovirus spreads via the fecal-oral route, often through contaminated hands or surfaces. The oral vaccine’s weakened virus can also be shed and mutate in under-vaccinated populations, leading to vaccine-derived cases. In 2023, vaccine-derived strains caused 524 cases globally, a decline from 881 in 2022.
Conclusion
The debate over the polio vaccine underscores the ongoing tension between vaccine skepticism and public health advocacy. While proponents stress the vaccine’s critical role in eradicating a deadly disease, detractors like Siri and ICAN raise questions about regulatory processes and safety standards. The outcome of the FDA’s review will have significant implications for public health policy and vaccine confidence.
This article was rewritten by JournosNews.com based on verified reporting from trusted sources. The content has been independently reviewed, fact-checked, and edited for accuracy, neutrality, tone, and global readability in accordance with Google News and AdSense standards.
All opinions, quotes, or statements from contributors, experts, or sourced organizations do not necessarily reflect the views of JournosNews.com. JournosNews.com maintains full editorial independence from any external funders, sponsors, or organizations.
Stay informed with JournosNews.com — your trusted source for verified global reporting and in-depth analysis. Follow us on Google News, BlueSky, and X for real-time updates.