World-First Gene Therapy Shows Promising Results in Hunter Syndrome
A three-year-old boy from California, Oliver Chu, has amazed medical experts after becoming the first person globally with Hunter syndrome to receive a pioneering gene therapy designed to halt the disease’s progression. The rare, inherited disorder, also known as MPS II, gradually damages the body and brain, often leading to death before the age of 20 in severe cases.
Oliver’s treatment represents a breakthrough: scientists in Manchester modified his own cells to produce an enzyme his body could not previously make. A year after the procedure, he is showing near-normal development, including cognitive and motor improvements, offering hope to families affected by this devastating condition.
Hunter Syndrome: Rare and Devastating
Hunter syndrome is caused by a genetic mutation that prevents the production of iduronate-2-sulfatase (IDS), an enzyme critical for breaking down large sugar molecules in cells. Without IDS, these molecules accumulate, causing damage to organs, bones, joints, and the nervous system. Symptoms typically appear around age two, including stiff limbs, short stature, delayed speech, and progressive cognitive decline. The condition almost exclusively affects boys, with an incidence of approximately one in 100,000 male births.
Until now, treatment options were limited. Enzyme replacement therapy, such as Elaprase, can slow some physical effects but does not cross the blood-brain barrier and cannot prevent cognitive decline. Treatments cost around £300,000 per patient annually.
The Gene Therapy Process
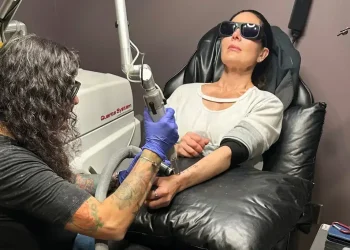
Oliver’s therapy began in December 2024 at the Royal Manchester Children’s Hospital. Doctors removed a portion of his stem cells using a machine designed for precise collection. These cells were then sent to Great Ormond Street Hospital in London for genetic modification.
In the lab, scientists inserted a functional copy of the IDS gene into a harmless virus, which served as a delivery mechanism. The virus then transferred the gene into Oliver’s stem cells. Importantly, the gene was modified to allow the enzyme to cross the blood-brain barrier, addressing both physical and neurological symptoms.
By February 2025, the gene-modified stem cells were returned to Manchester for infusion. Oliver received two infusions via a catheter in his chest, each containing approximately 125 million modified stem cells. The procedure was completed in minutes, and Oliver remained calm, watching cartoons during the treatment.
Early Signs of Improvement
By May 2025, follow-up testing indicated significant progress. Oliver displayed improved mobility, speech, and social engagement. Crucially, he no longer required weekly enzyme infusions, as his body had begun producing the missing IDS enzyme naturally.
His mother, Jingru, described the changes as “amazing,” while his father, Ricky, emphasized the rapid improvement in cognitive and physical abilities. Oliver’s older brother, Skyler, also diagnosed with Hunter syndrome, continues to receive conventional infusions but is not yet eligible for gene therapy targeting the brain.
Ongoing Monitoring and Future Plans
Oliver will continue regular check-ups every three months to monitor his enzyme production and neurological development. The clinical trial includes five boys from the US, Europe, and Australia, all of whom will be observed for at least two years. If successful, researchers plan to partner with biotech companies to make the therapy widely available.
Professor Simon Jones, co-leader of the trial, stressed cautious optimism: “Things are as good as they could be at this point, but we need to monitor carefully. We are encouraged by his progress in learning, mobility, and overall health.”
A Trial That Nearly Never Happened
The gene therapy originated from more than 15 years of research led by Professor Brian Bigger at the University of Manchester. Initially, a partnership with US biotech Avrobio faced setbacks due to poor results in a separate gene therapy trial and funding shortages, putting the first-in-human trial in jeopardy.
British charity LifeArc intervened with £2.5 million in funding, allowing the trial to proceed. Dr Sam Barrell, CEO of LifeArc, highlighted the challenges faced by patients with rare diseases, noting that 95% of such conditions have no effective treatments.
Hope for Families Affected by Hunter Syndrome
For the Chu family, the successful gene therapy has transformed daily life. Oliver now produces the missing enzyme, enjoys improved mobility, and engages fully with his surroundings. His parents hope Skyler may one day benefit from a similar treatment.
Ricky Chu reflected on the journey: “I would walk to the end of the earth, backwards, forwards, upside down, barefoot, to make sure my kids have a better future.”
The trial represents a landmark achievement in gene therapy, with potential applications for other rare genetic disorders, including MPS I (Hurler syndrome) and MPS III (Sanfilippo syndrome). Oliver’s story underscores the promise of genetic medicine in changing the outlook for children with previously untreatable conditions.
This article was rewritten by JournosNews.com based on verified reporting from trusted sources. The content has been independently reviewed, fact-checked, and edited for accuracy, neutrality, tone, and global readability in accordance with Google News and AdSense standards.
All opinions, quotes, or statements from contributors, experts, or sourced organizations do not necessarily reflect the views of JournosNews.com. JournosNews.com maintains full editorial independence from any external funders, sponsors, or organizations.
Stay informed with JournosNews.com — your trusted source for verified global reporting and in-depth analysis. Follow us on Google News, BlueSky, and X for real-time updates.