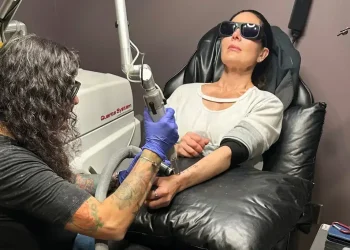
Unregulated Peptide Injections Surge as Wellness Trend Raises Safety and FDA Compliance Questions
The growing popularity of unapproved peptide injections—promoted across social media by wellness influencers, fitness personalities, and celebrities—has triggered concern among health experts and regulators. Marketed as shortcuts to improve muscle tone, boost metabolism, and slow aging, many of these compounds lack clinical data, FDA approval, and standardized manufacturing oversight.
Despite their rising visibility, scientists warn that many peptides have never undergone the rigorous human testing required to verify safety or effectiveness. As sales rise through online retailers and wellness clinics, questions continue to mount about potential risks, regulatory gaps, and the influence of celebrity endorsements.
A Growing Market With Minimal Oversight
Online stores now offer injectable peptide vials ranging from $300 to $600, while high-end wellness clinics provide in-office injections bundled with monthly membership programs that can exceed several thousand dollars. Such services are marketed as personalized longevity treatments, even though most products do not meet federal drug-approval standards.
Researchers caution that without clinical trials, the potential side effects—such as immune reactions, hormonal disruptions, or metabolic issues—remain unknown.
Understanding Peptides and Their Medical Uses
Peptides are short chains of amino acids that serve essential biological functions within the human body. They act as signaling molecules, hormones, and regulators of metabolic processes. Insulin—one of the most widely used peptide-based medications—helps manage blood glucose and supports the body’s energy balance. Similarly, GLP-1 drugs, used globally for diabetes and weight management, are based on gut-derived peptides that stimulate insulin release and regulate appetite.
These therapies are fully approved by the U.S. Food and Drug Administration (FDA), supported by decades of clinical research. But beyond these established medications, thousands of other peptides exist, and many have been studied only in animals or lab environments.
Dozens of these lesser-known peptides—such as BPC-157, thymosin alpha-1, and GHK-Copper—have become central to the wellness trend despite lacking formal evaluation in humans.
Why Peptides Have Become a Viral Wellness Trend
Although synthetic peptides have circulated in specialized medical fields for decades, their rise in mainstream wellness culture is recent. Influencers with large followings have embraced peptides as part of broader “biohacking” routines, often promoting them for anti-aging benefits, injury recovery, and general vitality.
Among online communities, “peptide stacks”—the combined use of multiple injectable compounds—are increasingly common. Researchers warn this practice magnifies the risk of unintended interactions.
Dr. Eric Topol of the Scripps Research Translational Institute has voiced concerns about multi-peptide regimens, noting that users often take “two, three, four different peptides” simultaneously, without guidance from controlled studies.
Celebrity endorsements have further accelerated interest. Media personalities have publicly discussed using BPC-157 for injury recovery, while actor Jennifer Aniston has described weekly peptide injections as part of her skincare routine and now represents a company selling peptide-infused supplements. Wellness practitioners acknowledge that such visibility significantly boosts consumer demand.
Yet some providers, responding to FDA warnings, have stopped offering peptides like BPC-157 due to regulatory scrutiny.
How the FDA Regulates Peptides
Under U.S. federal law, any substance intended to treat or prevent medical conditions must be regulated as a drug. This means most peptides—particularly injectable formulations sold online—are technically illegal without FDA approval.
The FDA classifies many peptides as biologics, which require stricter manufacturing controls because of their complexity and potential for contamination. To protect consumers, the agency has placed numerous peptides on a list of substances that compounding pharmacies should not produce due to safety concerns.
Some companies attempt to market peptides as dietary supplements, particularly when sold as capsules, powders, or gummies. However, the FDA requires supplements to contain only ingredients recognized as permissible under federal law, and most peptides do not qualify. Even when taken orally, experts note that peptides are unlikely to survive digestion, making their effects minimal or nonexistent.
Who Produces These Unapproved Peptides?
In the absence of FDA-approved manufacturers, many injectable peptides are produced by compounding pharmacies. These facilities are designed to create customized medications—such as alternative formulations of existing drugs—when no commercially available version meets a patient’s needs.
Compounding pharmacies are regulated primarily at the state level and do not undergo the same inspections or quality controls as FDA-supervised drug manufacturers. In recent years, many compounders moved into the market for GLP-1 medications amid national shortages. Although the FDA declared the shortage resolved earlier this year, some compounders continue producing alternative versions of GLP-1 drugs, sometimes adding ingredients such as vitamin B.
Legal experts note that financial incentives have encouraged some pharmacies to push boundaries. As attorney Nathaniel Lacktman observed, the sector had “never seen the monetary incentive” to intensify production until demand for peptides and GLP-1 alternatives surged.
This trend has drawn increased federal attention. The FDA has recently added more than two dozen peptides to an interim list of substances prohibited from compounding due to potential safety hazards.
Peptides and the Make America Healthy Again Movement
Peptides have also become a defining element of the Make America Healthy Again (MAHA) movement, led by U.S. Health Secretary Robert F. Kennedy Jr. Kennedy has repeatedly criticized FDA restrictions on peptides, arguing that the agency has stifled innovation and access to promising therapies.
Several figures associated with the movement—including wellness coach Gary Brecka and functional medicine author Dr. Mark Hyman—are prominent advocates for peptide use. Many in the peptide industry believe that Kennedy may seek to relax FDA oversight, potentially issuing a list of peptides that the government will permit without enforcement.
Public health experts caution that loosening regulations could expose consumers to compounds that lack safety testing, standardized dosing, or reliable manufacturing controls.
Weighing the Benefits Against the Risks
While early laboratory research suggests potential therapeutic uses for several peptides, scientists emphasize that animal studies cannot determine safety or effectiveness in humans. Without controlled clinical trials, dosage guidance, and long-term monitoring, the risks remain largely unknown.
Health authorities encourage consumers to consult licensed medical professionals and rely on FDA-approved treatments rather than turning to unregulated injections sold online or through loosely supervised wellness clinics.
As the popularity of peptides continues to grow, regulators, clinicians, and public health researchers warn that the industry’s rapid expansion must be matched by stronger evidence, clearer oversight, and a focus on consumer safety.
This article was rewritten by JournosNews.com based on verified reporting from trusted sources. The content has been independently reviewed, fact-checked, and edited for accuracy, neutrality, tone, and global readability in accordance with Google News and AdSense standards.
All opinions, quotes, or statements from contributors, experts, or sourced organizations do not necessarily reflect the views of JournosNews.com. JournosNews.com maintains full editorial independence from any external funders, sponsors, or organizations.
Stay informed with JournosNews.com — your trusted source for verified global reporting and in-depth analysis. Follow us on Google News, BlueSky, and X for real-time updates.