FDA Approves First At-Home Cervical Cancer Screening Device
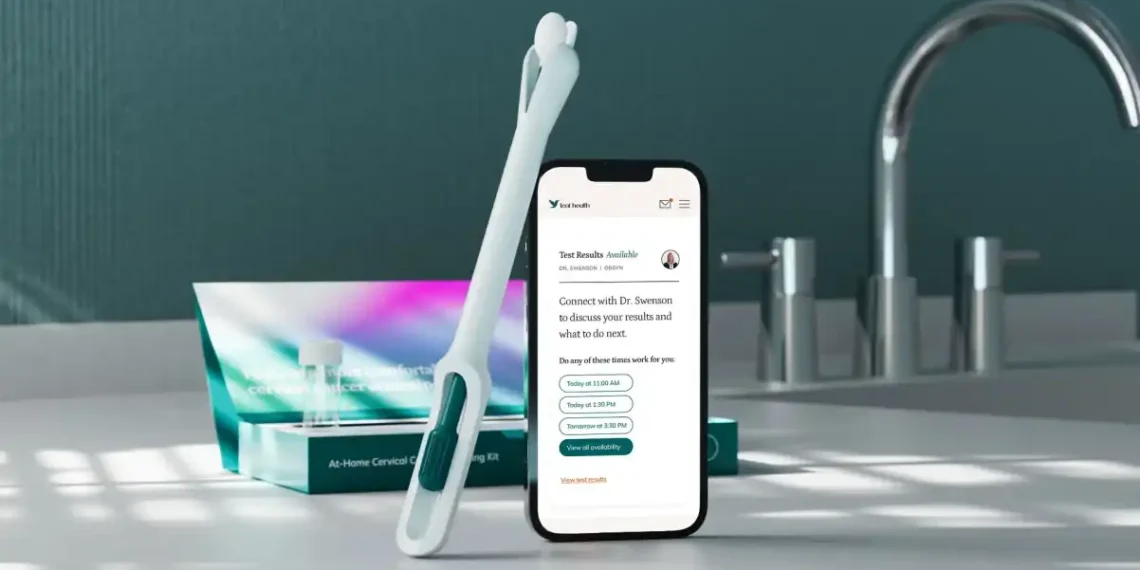
In a major step forward for women’s health, the U.S. Food and Drug Administration has approved the Teal Wand — the first at-home device for cervical cancer screening. This breakthrough means women may soon be able to skip the speculum and stirrups and screen for cervical cancer from the comfort of their own home.
A Game-Changer for Cervical Cancer Screening
Developed by Teal Health, the Teal Wand allows users to collect their own vaginal samples at home and send them to a lab for HPV testing. The device will be included in Teal Health’s upcoming at-home screening kit, which will be available by prescription starting this June — launching first in California, with plans to expand across the U.S.
“This is the same test, with the same accuracy as in-clinic collection — but now you can do it from home,” said Teal Health CEO Kara Egan. “It gives women more options, especially through telehealth, and increases access to essential care.”
How It Works
Here’s how the process unfolds:
- Request a Kit: Patients begin by visiting Teal Health’s website to request a kit.
- Telehealth Visit: A quick consultation with a provider results in a prescription.
- At-Home Collection: The patient uses the Teal Wand to collect a sample in private.
- Mail to Lab: The sample is sent to a lab and analyzed using Roche’s HPV testing platform.
- Results & Follow-Up: Results are reviewed by a clinician and shared with the patient. If needed, follow-up care is arranged.
Why It Matters
HPV (human papillomavirus) is the leading cause of cervical cancer, and early detection is critical. Yet millions of people in the U.S. are not up to date on their screenings. According to the CDC, about one in four adults fall behind on cervical cancer screening guidelines.
“This new test could be a game-changer,” said Dr. Ami Vaidya, co-chief of gynecologic oncology at Hackensack University Medical Center. “Some women avoid Pap smears due to discomfort or fear. Giving them a reliable at-home option could increase regular screenings, especially for those without easy access to a doctor.”
Support from Experts
The American Cancer Society praised the FDA’s decision.
“Most cervical cancers are found in people who were never screened or haven’t been screened recently,” said Dr. William Dahut, the organization’s chief scientific officer. “This at-home test adds a powerful tool in our fight against a potentially deadly disease.”
Understanding Cervical Cancer Screening
Cervical cancer screening typically involves:
- Pap tests (cytology): Detects abnormal cell changes in the cervix.
- HPV testing: Checks for the virus that can cause those changes.
The U.S. Preventive Services Task Force recommends:
- Women ages 21–29: Pap test every 3 years.
- Women ages 30–65: Either Pap test every 3 years, HPV test every 5 years, or both tests every 5 years.
Teal Health’s self-collection kit is designed to match the accuracy of these traditional methods while removing barriers like scheduling appointments or facing uncomfortable procedures.
What’s Next?
Teal Health is currently in talks with insurance providers to cover the cost of the kit. For those without insurance, pricing will be announced soon. A waitlist is already open on the company’s website.
With FDA approval now in place, the future of cervical cancer screening is looking more accessible — and more comfortable — than ever.
This article was rewritten by JournosNews.com based on verified reporting from trusted sources. The content has been independently reviewed, fact-checked, and edited for accuracy, neutrality, tone, and global readability in accordance with Google News and AdSense standards.
All opinions, quotes, or statements from contributors, experts, or sourced organizations do not necessarily reflect the views of JournosNews.com. JournosNews.com maintains full editorial independence from any external funders, sponsors, or organizations.
Stay informed with JournosNews.com — your trusted source for verified global reporting and in-depth analysis. Follow us on Google News, BlueSky, and X for real-time updates.