A blood test capable of identifying fragments of cancer DNA shows potential to detect more than 50 cancer types, including those without standard screening. Early results from a North American trial suggest the test may transform cancer detection and improve treatment success.
Breakthrough in Early Cancer Detection
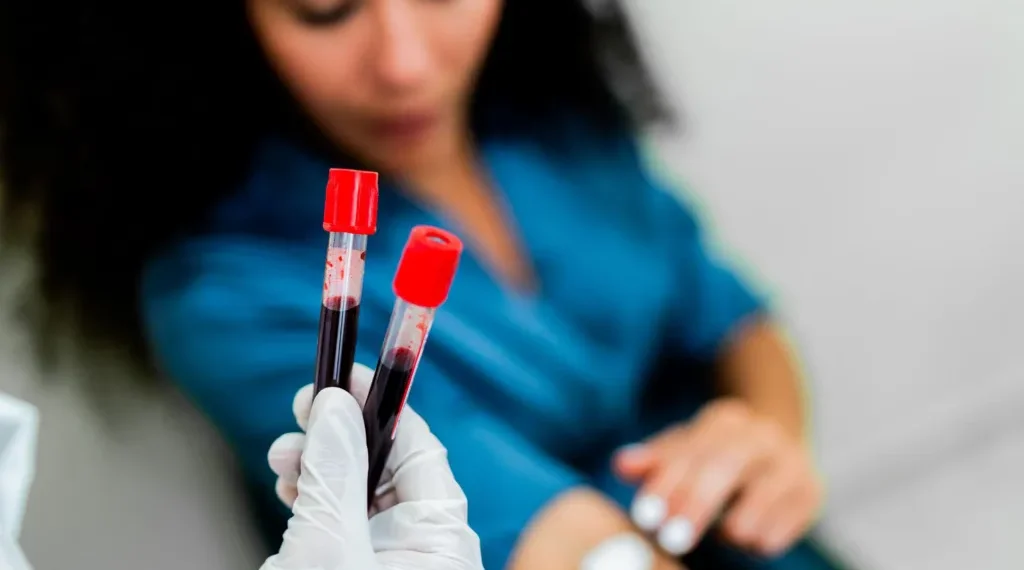
The Galleri blood test, developed by U.S.-based pharmaceutical company Grail, can detect fragments of cancerous DNA circulating in the bloodstream. It is designed to identify cancers at an early stage, when treatment is more likely to be effective and potentially curative.
In a trial involving 25,000 adults in the United States and Canada over one year, nearly 1% of participants received a positive test result. Subsequent medical follow-up confirmed cancer in 62% of these cases. Lead researcher Dr. Nima Nabavizadeh, associate professor of radiation medicine at Oregon Health & Science University, stated that the findings could “fundamentally change” the approach to cancer screening.
High Accuracy and Early Detection
The trial demonstrated that the Galleri test correctly ruled out cancer in over 99% of participants who tested negative. More than half of detected cancers were identified at an early stage, increasing the likelihood of successful treatment.
When combined with conventional screening for breast, bowel, lung, and cervical cancers, the test increased the total number of detected cancers seven-fold. Notably, three-quarters of detected cases were cancers for which no routine screening currently exists, including ovarian, liver, stomach, bladder, and pancreatic cancers.
The test accurately identified the origin of cancer in approximately 90% of cases, providing crucial information for timely and targeted treatment.
Expert Perspectives on Potential Impact
Sir Harpal Kumar, president of biopharma at Grail, described the results as “very compelling,” emphasizing that most cancer deaths occur because cancers are detected too late. “The goal is to shift to earlier detection, when we can use treatments that are more effective and potentially curative,” he said on BBC Radio 4.
However, some experts caution that earlier detection does not automatically translate into reduced mortality. Clare Turnbull, professor of translational cancer genetics at The Institute of Cancer Research in London, stressed the need for randomized studies with mortality as a primary endpoint to confirm the test’s real-world benefits.
Naser Turabi of Cancer Research UK added that careful evaluation is required to avoid overdiagnosis of cancers that may never have caused harm. He noted that the UK National Screening Committee will play a central role in reviewing evidence before any NHS adoption.
Ongoing Research and NHS Trials
The topline results from the North American trial are set to be presented at the European Society for Medical Oncology congress in Berlin, though full peer-reviewed data have not yet been published.
In England, a three-year NHS study involving 140,000 participants is underway, with results expected next year. The NHS has indicated that, if the findings are favorable, the Galleri test could be expanded to an additional one million people, potentially reshaping national cancer screening strategies.
Future Implications for Cancer Care
The Galleri test represents a potential leap forward in early cancer detection, particularly for cancers lacking established screening programs. Experts highlight its promise in identifying diseases when interventions are most effective, potentially reducing the burden of late-stage diagnoses.
At the same time, further research is essential to understand the balance between early detection and overdiagnosis. Ongoing trials and rigorous peer-reviewed studies will be critical in determining how the test may be integrated into routine clinical practice.
▶ Stay informed with JournosNews.com — your trusted source for verified global reporting and in-depth analysis. Follow us on Google News and BlueSky for real-time updates.
This article was rewritten by JournosNews.com based on verified reporting from trusted sources. The content has been independently reviewed, fact-checked, and edited for accuracy, neutrality, tone, and global readability in accordance with Google News and AdSense standards.
All opinions, quotes, or statements from contributors, experts, or sourced organizations do not necessarily reflect the views of JournosNews.com. JournosNews.com maintains full editorial independence from any external funders, sponsors, or organizations.
Stay informed with JournosNews.com — your trusted source for verified global reporting and in-depth analysis. Follow us on Google News, BlueSky, and X for real-time updates.